Initial report of extraperitoneal pedicle dissection in deep inferior epigastric perforator flap breast reconstruction using the da Vinci SP
Article information
Abstract
The deep inferior epigastric perforator (DIEP) flap has been widely used for autologous breast reconstruction after mastectomy. In the conventional surgical method, a long incision is needed at the anterior fascia of the rectus abdominis muscle to obtain sufficient pedicle length; this may increase the risk of incisional hernia. To shorten the incision, several trials have investigated the use of endoscopic/robotic devices for pedicle harvest; however, making multiple additional incisions for port insertion and operating in the intraperitoneal field were inevitable. Here, we describe the first case, in which a DIEP free flap was successfully made using the da Vinci SP model. Our findings can help surgeons perform operations in smaller fields with a single port in the extraperitoneal space. Moreover, this method is expected to lead to fewer donor-related complications and faster healing.
INTRODUCTION
Breast reconstruction is widely performed to achieve full recovery after mastectomy. Among the two reconstruction types (i.e., autologous and implant-based reconstruction), autologous reconstruction using abdominal tissue is considered the gold standard [1]. During the harvest of an abdomen-based flap, it is necessary to obtain a sufficient pedicle length, which requires surgeons to make a sufficiently long incision along the anterior fascia of the rectus abdominis muscle. However, this technique might be associated with a higher risk of hernia and injury to the muscle and the nerve [2].
There have been several trials of endoscopic/robotic devices to harvest the pedicle using shorter incisions [3-6]. Although shortening of a single incision was possible with the da Vinci Xi model, multiple additional incisions for port insertion were needed. Moreover, the operations were performed in an intraperitoneal field, which poses a risk of unexpected morbidity, as plastic surgeons do not frequently perform surgery in the intraperitoneal space.
A new robot, the da Vinci SP model (Intuitive Surgical Inc., Sunnyvale, CA, USA), which allows surgeons to operate in a smaller field with a single port, was recently released. Through this port, a smaller camera can be inserted together with three arms for operation, thereby enabling surgeons to operate in a smaller field. Here, we describe the first case, in which a deep inferior epigastric perforator (DIEP) free flap was successfully performed using this model.
CASE
Patient selection
A 58-year-old female patient was diagnosed with right breast cancer. She had no medical or surgical history. She decided to undergo robot-assisted mastectomy in order to avoid having a scar over the anterior breast mound. Moreover, she preferred to have a short-incision scar. She was also reluctant to undergo implant insertion due to fears of the risk of infection after insertion of a foreign body. The course of the perforators of the deep inferior epigastric artery was checked on preoperative angiographic computed tomography. The general surgery team performed robot-assisted nipple-sparing mastectomy of the right breast with sentinel lymph node biopsy using the da Vinci SP model, followed by immediate reconstruction with a DIEP free flap.
Surgical technique
A 4- to 5-cm vertical incision for robot-assisted mastectomy was made on the lateral breast, in a location that could be hidden by the patient’s upper arm. To protect against maceration of the incision margin during mastectomy, a wound protector was used. Therefore, an incision of at least 4–5 cm was required. Moreover, 12 mmHg of CO2 gas was insufflated during robot-assisted mastectomy to create a surgical space.
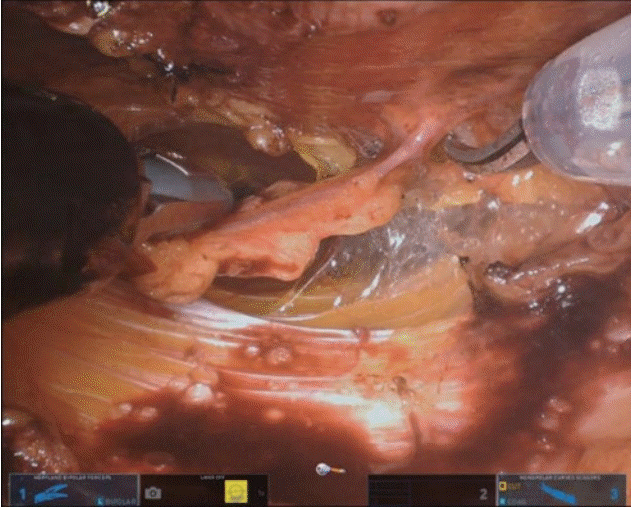
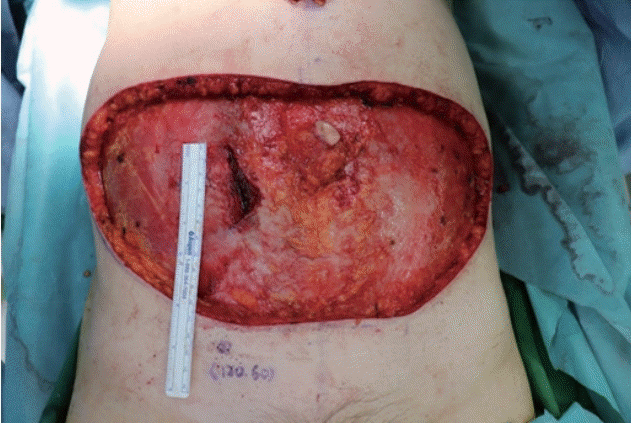
The skin incision on the abdomen was performed in a similar manner to the conventional DIEP free flap procedure. Moreover, control of the inframammary fold and preparation of the thoracodorsal vessels at the breast pocket were performed similar to the conventional method. To minimize the fascial incision, dissection to locate the perforator vessel was only performed from the lateral side. As we used two perforators in this surgery, intramuscular dissection of the perforator was performed manually. Interestingly, an approximately 5-cm fascial incision was necessary for this procedure. Dissection was performed up to the starting point of the submuscular course of the pedicle, and the incisional point for the new umbilicus was designed. After making an incision at the putative neo-umbilicus location, a single port of the da Vinci SP model was inserted (Fig. 1). At the most medial end of the ipsilateral rectus muscle, a fascial incision was performed, and the trocar was inserted below the incision. The robot was docked and advanced to the cephalic endpoint of manual dissection, and the fascial incision was temporarily sutured with Vicryl #2-0 sutures. CO2 gas was insufflated up to a pressure of 8 mmHg to obtain a surgical view. Further dissection of the pedicle was continued using Maryland bipolar forceps and monopolar scissors (Fig. 2). After a sufficient length of perforator was dissected, robot dissection was temporarily stopped, and the flap was fully detached from the abdominal fascia. Then, using the robot, the perforator vessels were ligated with clips, and the flap was elevated freely. The harvested flap was inserted at the breast pocket, and microanastomosis of the DIEP artery and vein with the thoracodorsal artery and vein was performed manually. The patient was seated, and after checking the aesthetic appearance and balance of the breasts, the flap was fixed (Fig. 3).
Trocar insertion at the putative location of the neo-umbilicus. (A, B) Submuscular pedicle dissection was performed by robot arms. Resection of zone 4 and de-epithelization of the flap was performed before flap detachment.
Results
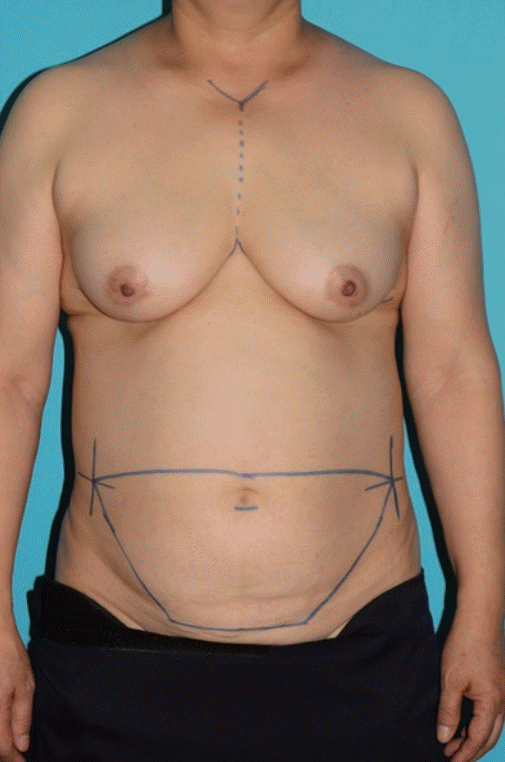
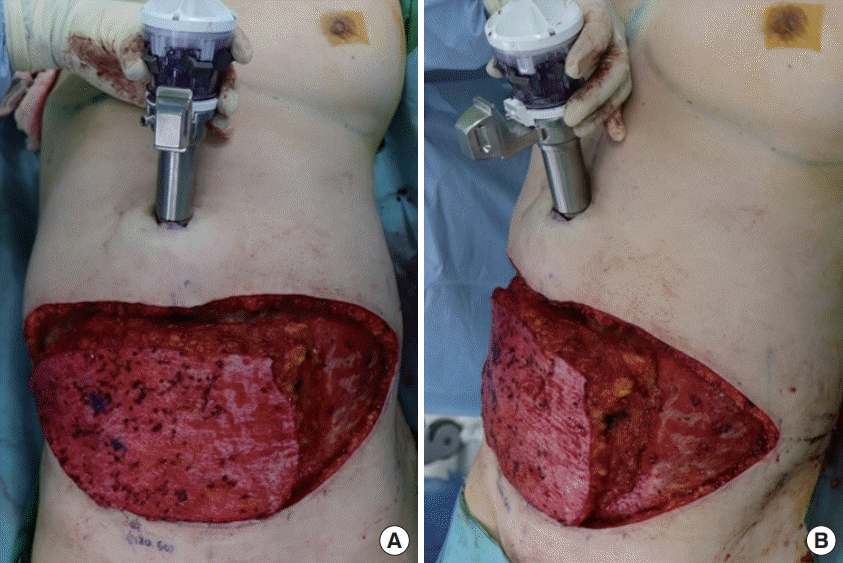
There were no postoperative complications, and the patient was discharged 1 week postoperatively. A comparison of a preoperative image (Fig. 4) and a 3-month postoperative image (Fig. 5) showed that it was possible to maintain a balanced breast with an esthetic shape. Approximately 7 months have passed after surgery, and no specific complication has been noted during routine follow-up examinations at the outpatient department of our hospital.
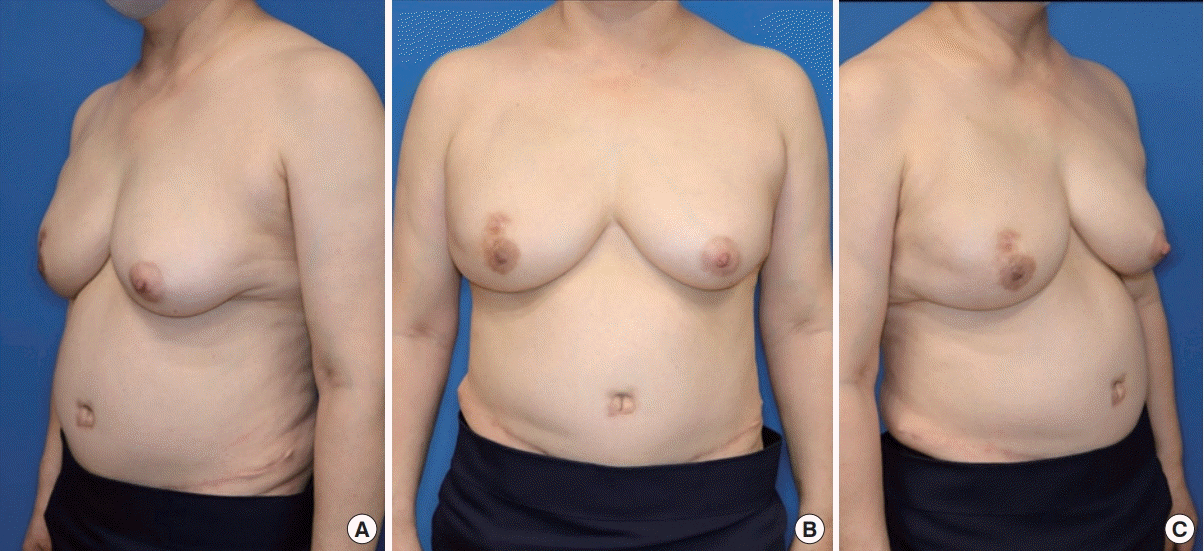
The patient’s breast at 3 months postoperatively. (A-C) The neo-umbilicus scar, which was made by the trocar insertion site, was not different compared to that after performing the conventional transverse rectus abdominis muscle free flap and deep inferior epigastric perforator free flap operation from an esthetic point of view.
DISCUSSION
When performing dissection for a DIEP free flap, surgeons may consider the advisability of performing a long fascial incision. Although it is easier to perform an operation through a long incision, there is a higher risk of injury to the rectus muscle and intercostal nerve, which in turn can lead to incisional hernia or weakening of the abdominal fascia [7]. However, when a short incision is made, there is a limited working space, which makes the operation difficult, thereby leading to increased injury risk. Once a pedicle is dislodged from the rectus abdominis muscle, it is difficult to place it back. Therefore, if it is possible to dissect the pedicle only in the submuscular space, we should avoid causing an injury to the rectus muscle and the anterior fascia. Many surgeons already have noticed this and tried to dissect the pedicle using a robotic or laparoscopic device. However, in these trials, the surgeons used a bulky device (da Vinci Xi model), which required several ports. Moreover, another issue was the fact that the robotic arm had to be docked in the intraperitoneal space; this was unfamiliar territory for plastic surgeons, as they usually perform operations in the extraperitoneal space
The newly developed da Vinci SP model only needs a single 2.5-cm diameter port for the entry of four arms, including an arm with a camera. This enables surgeons to operate in a smaller space, which is appropriate for pedicle dissection for a DIEP free flap. To our knowledge, this is the first report of successful pedicle dissection in the extraperitoneal space using this device. To date, only the performance of nipple-sparing mastectomy using the same robot by general surgeons has been reported. Based on our findings, we conclude that the da Vinci SP model is a good candidate for pedicle dissection of the DIEP free flap and can be used in such cases.
For minimally invasive surgery in the future, some additional points should be considered. First, we inserted the port directly beneath the fascia after making an incision in the anterior fascia of the rectus abdominis muscle. However, the presence of adhesions between the rectus abdominis muscle and the fascia would make it difficult to perform this procedure. Therefore, it seems to be safer to insert the port after moving the rectus abdominis muscle to the lateral side. Moreover, if it is possible to perform intramuscular dissection with a robot, it would be better to perform the entire procedure using a robotic device. At the end of the dissection, as the pedicle was moved inferiorly, the robotic device had to be tilted. This caused the robot device to increase the pressure on the patient’s face and sternum. By installing an additional joint and making a curved robotic arm, these problems can be solved.
By using the da Vinci SP model, we can reconstruct the breast using a DIEP free flap with a minimal fascial incision. This technique is expected to reduce donor-related complications and promote faster healing.
Notes
Conflict of interest
Seung Yong Song is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Ethical approval
The study was approved by the Institutional Review Board of Severance Hospital (IRB No. 4-2021-1462) and performed in accordance with the principles of the Declaration of Helsinki. The informed consent was waived because this design is a retrospective study.
Patient consent
The patient provided written informed consent for the publication and the use of her images.
Author contribution
Conceptualization: DW Lee, HS Park, DH Lew, TS Roh, SY Song. Data curation: YR Jeon. Formal analysis: YR Jeon. Methodology: YR Jeon. Project administration: SY Song. Visualization: SY Song. Writing - original draft: JH Jung. Writing - review & editing: YR Jeon, SY Song. All authors read and approved the final manuscript.